1. Objective
To
achieve and maintain inert atmosphere using nitrogen (N2) inertization in the
storage tank, ensuring safe storage conditions and preventing the risk of
combustion or oxidation of stored materials.
2. Guidelines Used
·
API 2000 Venting
Atmospheric and Low-Pressure Storage Tanks
·
NFPA 69
·
Normal Venting:
The venting required because of operational requirements or atmospheric
changes.
·
Nm3/h:
Normal cubic meters of air or gas per hour at a temperature of 0°C and pressure
of 1.014 bar.
·
Thermal inbreathing:
The movement of air or blanketing gas into a tank when vapors in the tank
contract or condense as a result of weather changes conditions (e.g., a
decrease in atmospheric temperature).
· N2
Inertization concept
The volume of
nitrogen gas required to inertize the tank during its operation depends on the
volume of inbreathing gas while the liquid is moved out of the tank and the
vapours condense or contracts in tank due to atmospheric temperature changes.
N2 inertization of tanks is done to provide inert atmosphere in storage tanks
storing flammable, combustible or reactive liquids. The volume of N2 required
under normal operating conditions is almost equal to the volumetric rate of
liquid discharge from the tank. However, due to sudden atmospheric changes and
temperature drops the vapour present in storage tanks contracts and condense
leading to vacuum and more Nitrogen requirement to fill up the vacuum space
generated. This factor is covered under thermal breathing requirements of tank.
As per API, calculations for thermal breathing requirements is based on
temperature drop of 37.8 °C within 1 hour.
As per API 2000:
Venting Atmospheric and Low-Pressure Storage Tanks, design of nitrogen
inertization system depends on the total normal inbreathing capacity which is
the sum of the inbreathing requirements for liquid movement and thermal effect.
During inbreathing nitrogen is provided instead of air to achieve an inert
atmosphere withing the storage tank. The Nitrogen supply line is connected to
the Storage Tank via Breather valve. As the liquid in the tank is pumped out or
drained or due to contraction or condensation of vapours inn storage tanks,
vacuum will be created which will trigger breather valve to open and let
nitrogen gas to occupy the vacuum space. The tank will be maintained at
atmospheric pressure with help of breather valve opening to provide nitrogen to
protect the tank against any vacuum while maintaining inert conditions.
· Process
Calculation for N2 Inertization
The following
sequence of activities is implemented during the N2 inertization calculation
for inbreathing of storage tanks only.
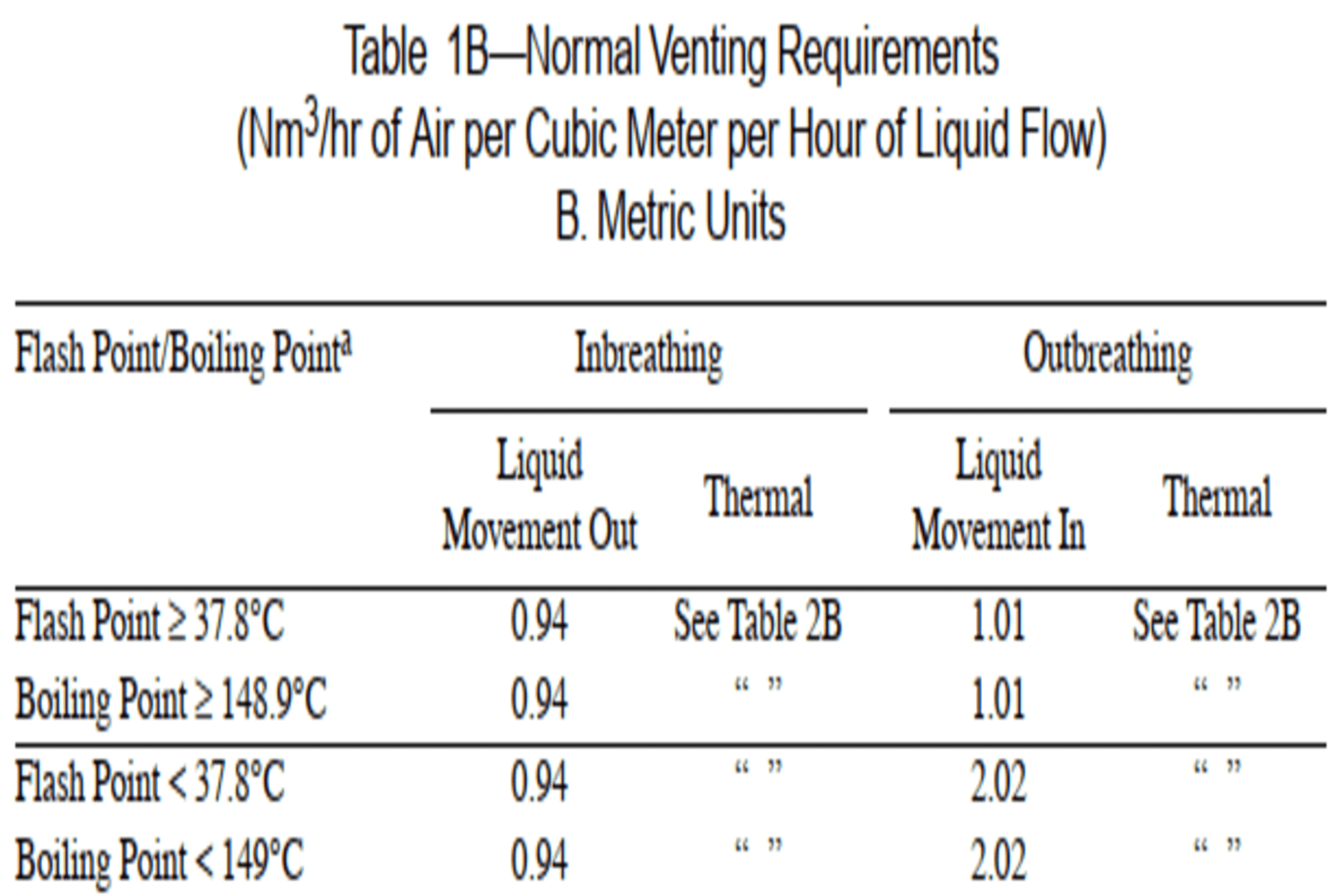
1. Calculation of inbreathing requirements is carried out from API 2000 Table 1B—Normal Venting Requirements. And Table 2B —Requirements for Thermal Venting Capacity.
i.
Determine the Flash
Point or Boiling Point of Liquid that is stored in the Storage Tank. If both
the flash point and boiling points are known then use flash point. On the basis
of flash point of liquid that is stored in tank choose an appropriate factor
under liquid movement out under inbreathing as per Table-1B.
ii.
Determine pump
flowrate responsible for Liquid Movement Out of Tank. The multiplication of
factor chosen in step (i) and pump flow rate shall give the Inbreathing
requirement for Liquid Movement Out of tank.
Example
calculation:
·
Flash Point of Liquid
Stored In tank = 9.7 °C.
·
Inbreathing
Requirement per m3/hr of Liquid Flow = 0.94 Nm3/hr
·
Amount of Liquid Flow
= 5 m3/hr
· Inbreathing Requirement for Liquid Flow of 5m3/hr = 0.94*5 Nm3/hr = 4.7 Nm3/hr.
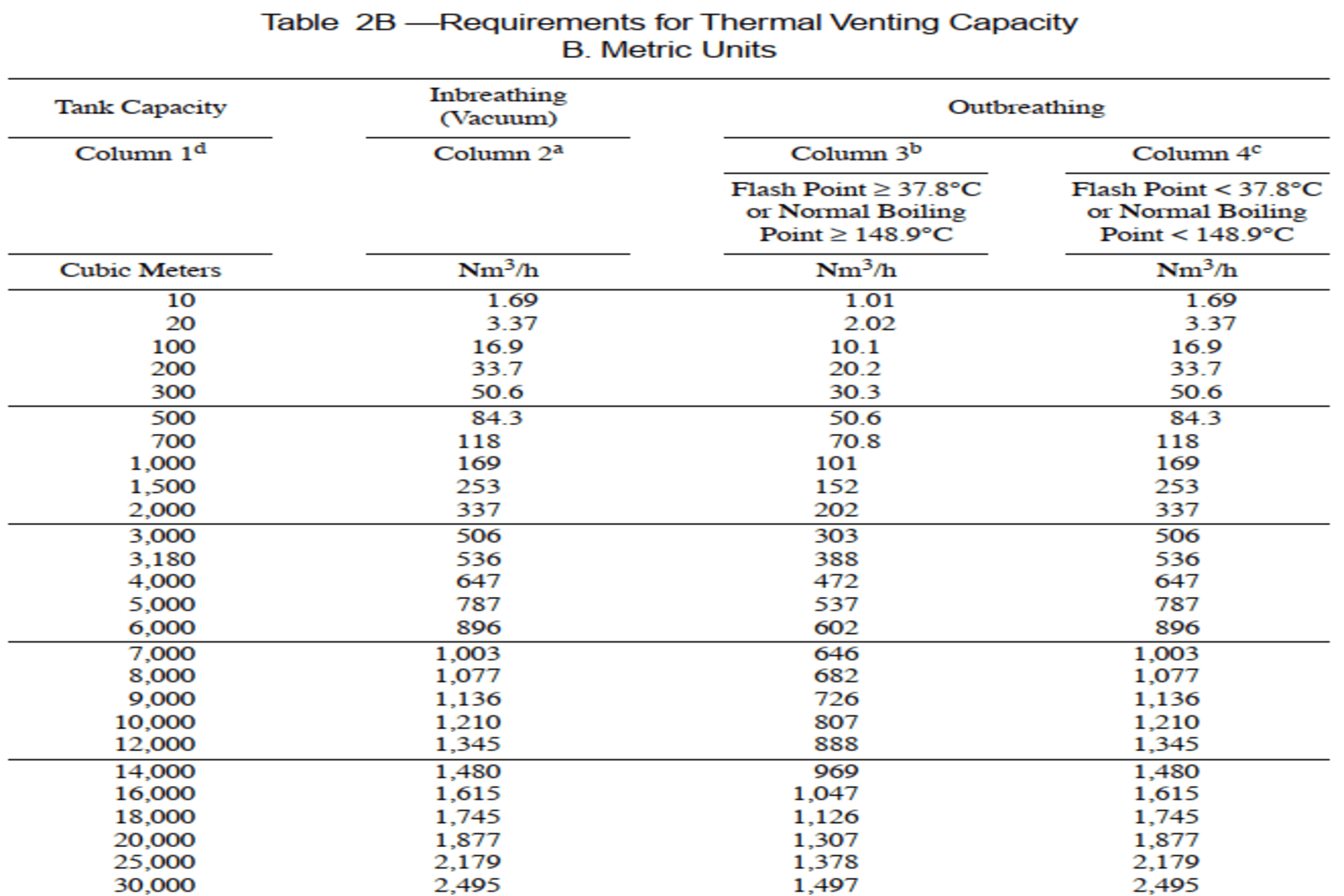
i.
Thermal Inbreathing
requirements are based on Tank Capacity. Determine Tank Capacity and its
Inbreathing requirement as per column 2a, Table-2B
ii.
For Intermediate
values of tank capacity, use interpolation method to find Inbreathing
requirement.
Example calculation:
·
Tank with capacity of
25kl = 25m3.
·
25m3
according to table is more than 20m3 and less than 100m3.
·
Unit Inbreathing
requirement between 20m3 and 100m3 is (16.9-3.37)/(100-20)=0.169125
·
Inbreathing
requirement for 5m3 = (0.169125*5) = 0.845625
· Inbreathing Requirement for 25m3 (20m3+5m3) = (3.37+0.845) = 4.215625 Nm3/hr
2.
The inbreathing
volume due to normal breathing requirements will give volume of nitrogen
required when liquid is pumped out of the tank and thermal breathing
requirements will give volume of nitrogen required due to temperature changes.
3.
Under normal
operating conditions nitrogen requirement will be calculated as in Step 1,
Normal Inbreathing requirement due to liquid movement out and Summation of both
the inbreathing requirements that is normal and thermal to get maximum
inbreathing volume of Nitrogen which may arise if temperature drops by 37.8 °C
within 1 hour during liquid outflow from the tank.
4.1.
Data
Requirement
Ø Equipment
P&IDs
Ø Equipment
Specification sheet
Ø Pump
Details like Head, Flow rate
Ø MSDS
of Chemicals
Ø Nitrogen upstream Details and Nitrogen Purity Level
4.2.
Project
Deliverables
Table 1: N2 Inertization Study
|
Sr. No. |
Document Deliverable |
|
1.
|
Executive Summary |
|
2.
|
N2 Inertization Methodology |
|
3.
|
Assumptions |
|
4.
|
|
|
5.
|
Conclusion |
|
6.
|
Site observation Details |
|
7.
|
Reference |
|
8.
|
N2
Inertization Calculation Worksheet |
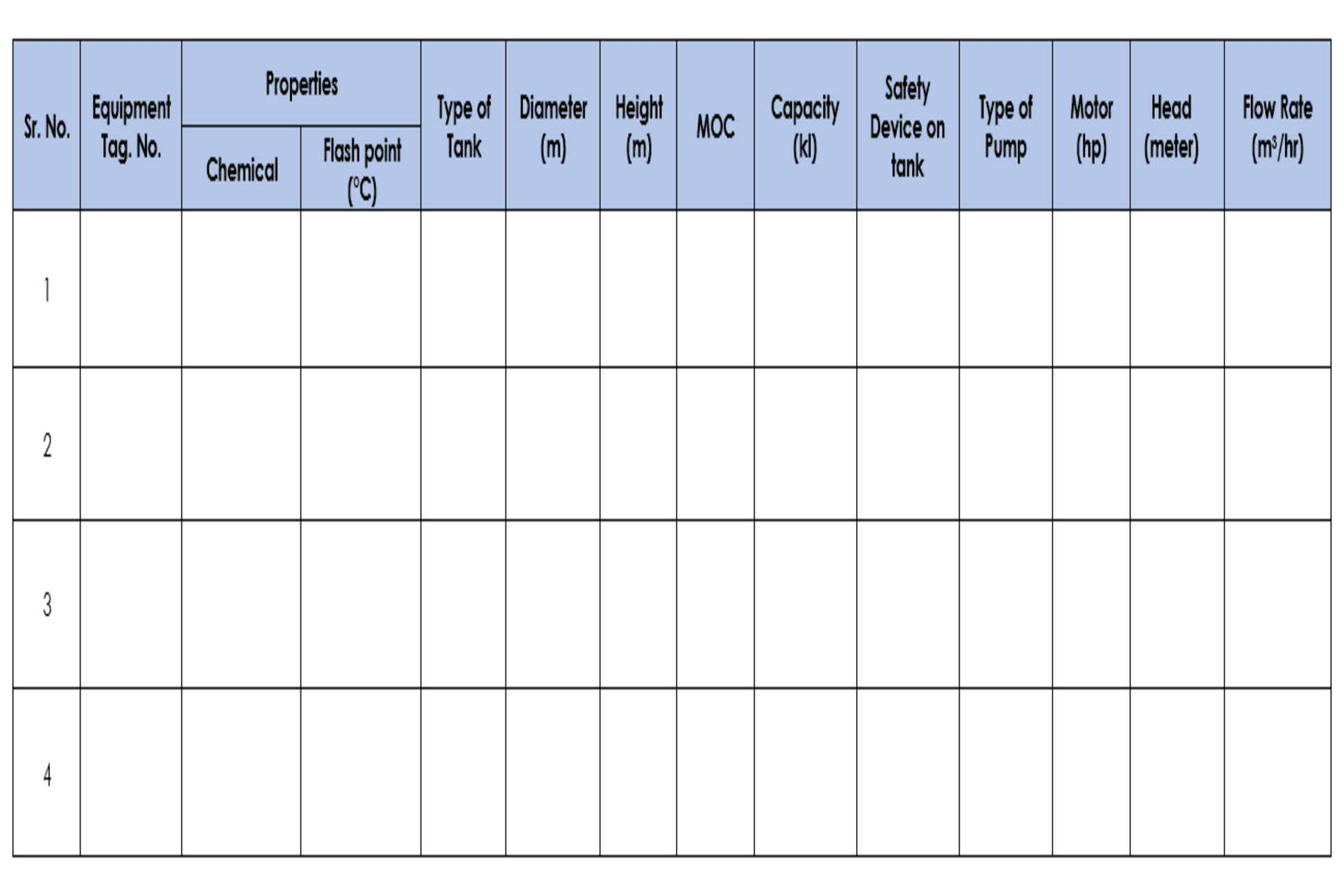
4.3 Site Visit Details Format
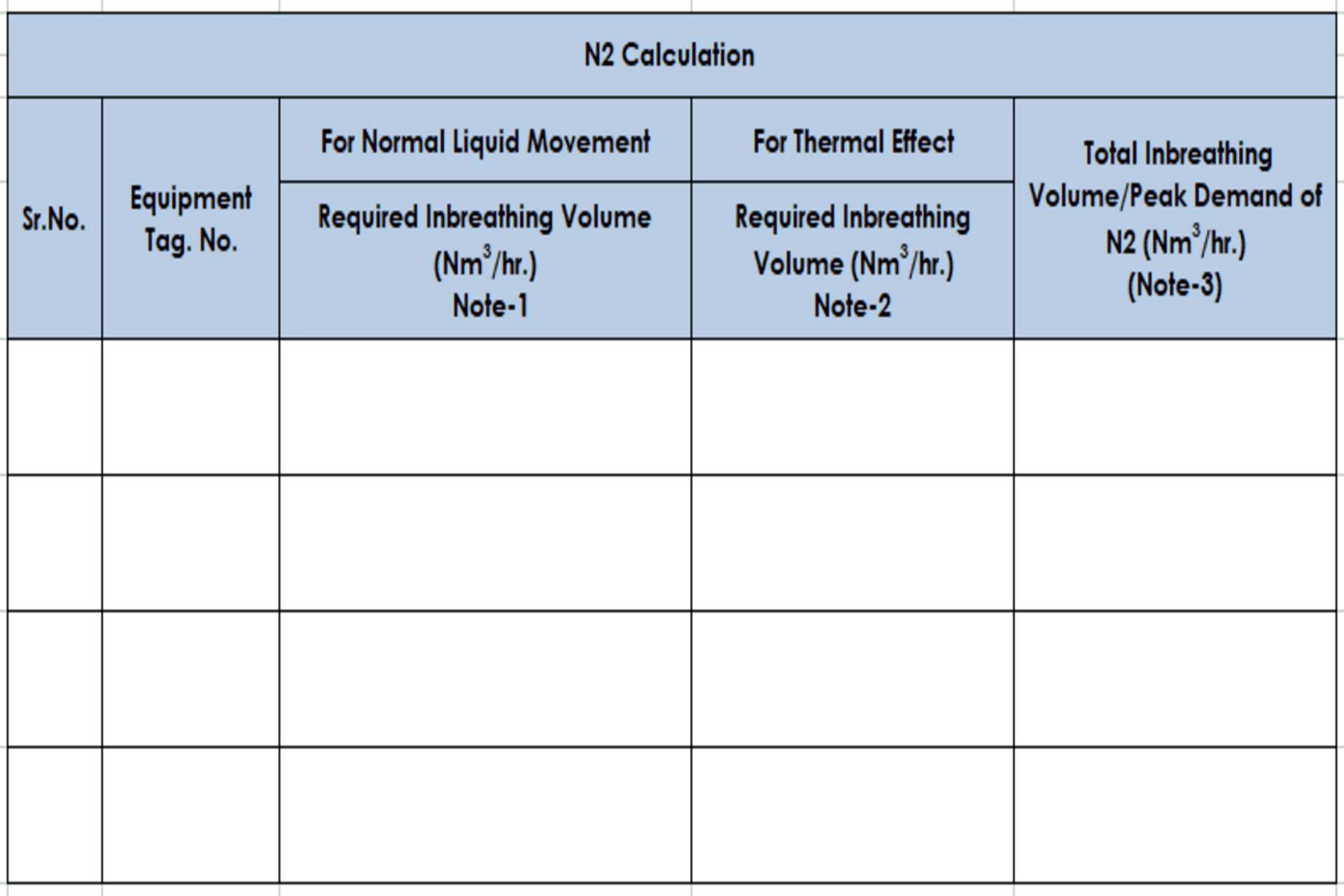
4.4 Vent
Sizing Calculation Worksheet Format
4.5 Software Used
The N2
Inertization calculation is done using Microsoft Excel.









